Companies are investing billions to make their data science operations robust and up to date with the latest technological advancements. But are they maximizing their data science investments to enhance decision-making? How often are decisions based on naive data assumptions? Do models adapt and reorient when underlying business assumptions change?
Answering these questions requires some introspection. Unfortunately, data science operations today rely heavily on historical summarized data to predict future outcomes, assuming the future will mirror the past. Because of this perspective, companies not only work hard to identify past trends, but they often rely too heavily on them to help guide decisions for the future of their business.
Human instinct craves certainty and clings to predictive models built on past trends. However, this breeds hubris. It offers an illusion of control, tempting analysts to over-plan and underestimate the inherent variability of the future. This ‘lazy mind working’ traps us in planning fallacies, shackling us to the past and blinding us to the emerging possibilities. In more sordid situations, this leads us to make decisions which are counterproductive for the business, giving data science teams a bad rep for their innovation solutions.
In the pharmaceutical industry, planning fallacies cast a long shadow, particularly in clinical trials and launch plans. Optimistic assumptions about site performance, patient recruitment, healthcare professional (HCP) writing behavior, marketing effectiveness and underestimating uncertainties from regulatory hurdles often lead to overestimations, leaving companies scrambling to play catch up to ever-shifting deadlines. Each day of delay comes with a hefty price tag, underscoring the limitations of historical, predictive models that do not account for the complex interaction between different entities. This overreliance on the patterns of the past repeating itself creates a dangerous blind spot, leaving companies vulnerable to the unpredictable reality of drug development and launch.
Linear Solutions Fail In Complex Problem Spaces
The Human mind derives power from certainty in predictions. However, true power lies not in clinging to predictions, but in embracing the empowering unknown. The hubris of data science needs a humility check from Complexity Sciences. The mindset shift allows one to move beyond mere prediction and planning towards adopting an OODA (Observe, Orient, Decide, and Act) approach. By embracing the cyclical nature of decision-making in a complex world, we trade hubris for humility, unlocking the true potential of data-driven action.
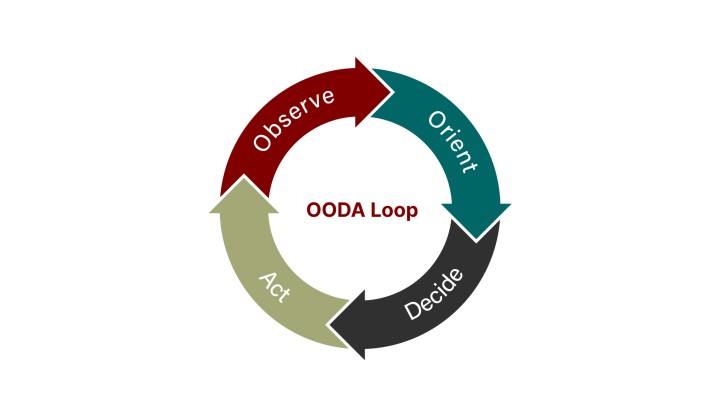
By acknowledging the inherent limitations of forecasting the future, we open ourselves to tools like prescriptive analytics. Instead of simply predicting what might happen, these tools help us navigate uncertainty by suggesting optimal actions for a range of possible scenarios.
Complex Systems In Healthcare
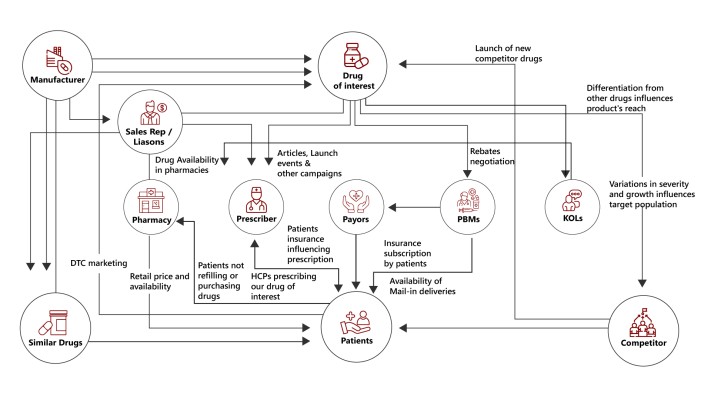
Creating and using Decision Support Systems grounded in complexity science can transform clinical trial operations and commercial launch strategies in pharma companies. The complexity of interactions within the healthcare ecosystem requires a sophisticated understanding beyond linear predictions. Complexity science can provide us with tools to help map the interactions of all the components within trial operations and commercial launch using complexity maps.
Complexity maps offer a powerful decision-making framework, acting as both a visual representation of the complexity of the problem space and also as a blueprint for designing decision engines for prescriptive analytics. Representing complexity can help decision makers see the interactions between the agents and influencers of a particular system, which in the case of clinical trials or a commercial launch would be patients, HCPs, regulators, pharma manufacturers and competitors. This enhanced understanding allows you to make informed decisions and confidently design interventions that target the heart of the problem. It can also help decision makers find and address vulnerabilities in the system, potentially saving big in terms of cost and time.
Simulate What-if Scenarios
Disruptive events that can significantly alter the progress of clinical trials or commercial launches cannot be predicted. But they can be used as a stress-test mechanism to simulate certain “what-if” worst-case outcomes and build a muscle for respond versus react. Here are some scenarios that can be designed as interventions to simulate “what if” cases. These scenarios can be simulated in any phase from trial to commercial launch.
- Black Swan Events: Imagining the unthinkable such as unforeseen regulatory policy changes and other external disruptions.
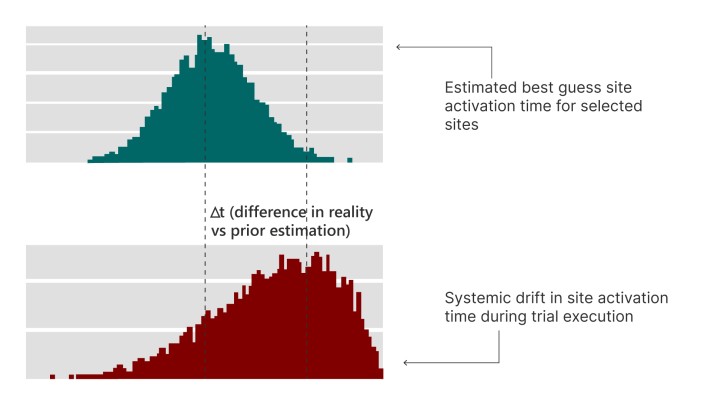
- Systemic Drift: Not all disruptions are dramatic. Sometimes, small delays in budget negotiations, regulatory backlogs, or delayed site activation can compound and throw off your timeline.
- Dynamic Changes: Clinical trials or Commercial Launches are rarely static. Changes in the site portfolio, addition of rescue sites, or fluctuations in patient enrollment rates can alter the trial progress. Changes in the formulary approvals, label expansions, new effectiveness information can alter the launch progress.
- Agent Behavioral Changes: Human behavior adds another layer of complexity. Changing Patient and investigator preferences, performance, or motivation could lead to delays or recruitment challenges. Similarly, changing HCP preferences and familiarity with Pharma brands, can spell danger for Commercial launch.
- Exogenous Changes: The competitive landscape can shift quickly. A sudden influx of competitors or changes in the eligible patient pool can impact a trial’s feasibility or a launch’s success.
Simulating these scenarios and a counterfactual thinking process can offer the insight necessary to ride out any potential storms, ultimately ensuring life-saving treatments reach patients faster and more reliably. This shift from “what will happen” to “what if this happens” empowers us to navigate uncertainty with greater agility and resilience. Simulation could also help identify potential bottlenecks before they become roadblocks, adjust timelines, and reallocate resources as needed. It’s not about predicting the future, but rather about being prepared for curveballs.
Interventions Are Necessary
Through a comprehensive approach incorporating scenario planning, pharmaceutical companies can significantly improve their adherence to trial and launch timelines, achieving cost savings and gaining a competitive advantage. The shift towards prescriptive solutions based on complexity sciences represents a vital strategic pivot for pharmaceutical companies. By embracing complexity, organizations can move beyond the limitations of predictive analytics, positioning themselves to respond more effectively to unpredictable future challenges, leading the way to more adaptable, resilient decision-making frameworks.
About the Author
Tanmay Sengupta , Business Unit Head at Mu Sigma, collaborates with Fortune 500 companies in Pharma, Medical Devices, and High Tech to establish a programmatic approach to decision-making.


