Imagine a scenario where an AI model, trained on incomplete or inaccurate data, misguides a pharmaceutical company in predicting adverse drug reactions. The result? A new medication, expected to save lives, instead causes unforeseen complications, leading to a mass recall and patient harm. Studies show that a majority of AI models fail due to poor data quality, underscoring the critical risks poor data poses in healthcare.
In the pharmaceutical industry, where data affects patients’ lives, the stakes are incredibly high. Real-World Evidence (RWE) studies offer invaluable insights into patient experiences, drug efficacy, and long-term safety outcomes. However, these studies are heavily reliant on data from a variety of sources, much of which are unstructured—clinical notes, patient feedback, and records from external databases. Unstructured data poses significant challenges for analysis and utilization unless properly governed and managed.
Generative AI (GenAI) is transforming how unstructured data is handled in RWE studies. By effectively processing, categorizing, and structuring unstructured data, GenAI enhances the accuracy of AI models, leading to better decision-making and improved patient outcomes.
The Challenge of Unstructured Data in RWE Studies
Unstructured data, representing around 80% of healthcare data, presents a formidable opportunity. Conventional data analysis tools falter with unstructured data, leading to incomplete or inaccurate AI models. Manual reviews are labor-intensive and error-prone, while even advanced natural language processing (NLP) tools often miss critical context due to the complexities of medical jargon and varying data formats. It results in flawed AI models that can lead to misguided decisions in drug development and patient care
Clinical notes, patient feedback, and external reports often come in formats that traditional methods struggle to process, which makes the need for faster tools to derive actionable insights and improve the quality of AI models is an area that is still in its infancy and growing rapidly.
GenAI Opportunity for Pharma Executives
Pharmaceutical executives are acutely aware of the pressures to reduce time-to-market, enhance regulatory compliance, and foster innovation. In a landscape where speed and precision are paramount, implementing Generative AI (GenAI) offers transformative benefits that align directly with these strategic goals.
Accelerating Time-to-Market
GenAI’s ability to process and structure unstructured data significantly reduces the time required to derive actionable insights from Real-World Evidence (RWE) studies. By automating the analysis of clinical notes, patient feedback, and external reports, GenAI ensures that AI models are more accurate and efficient, thereby accelerating drug development cycles. The reduction in time-to-market not only boosts competitiveness but also ensures that life-saving medications reach patients faster.
Enhancing Regulatory Compliance
Compliance with stringent regulatory frameworks like the FDA, EMA, and GDPR is non-negotiable in the pharma industry. GenAI enhances transparency and traceability in data processing, making it easier for companies to meet regulatory requirements. By generating auditable AI models and maintaining high data quality, GenAI helps pharma companies navigate complex compliance landscapes while safeguarding patient data.
Driving Innovation
Innovation in drug discovery often hinges on the ability to identify patterns and insights that are not immediately apparent. GenAI’s deep learning capabilities unlock new opportunities by uncovering hidden correlations in unstructured data, which empowers pharma companies to explore novel therapeutic areas, develop personalized treatments, and enhance overall R&D productivity.
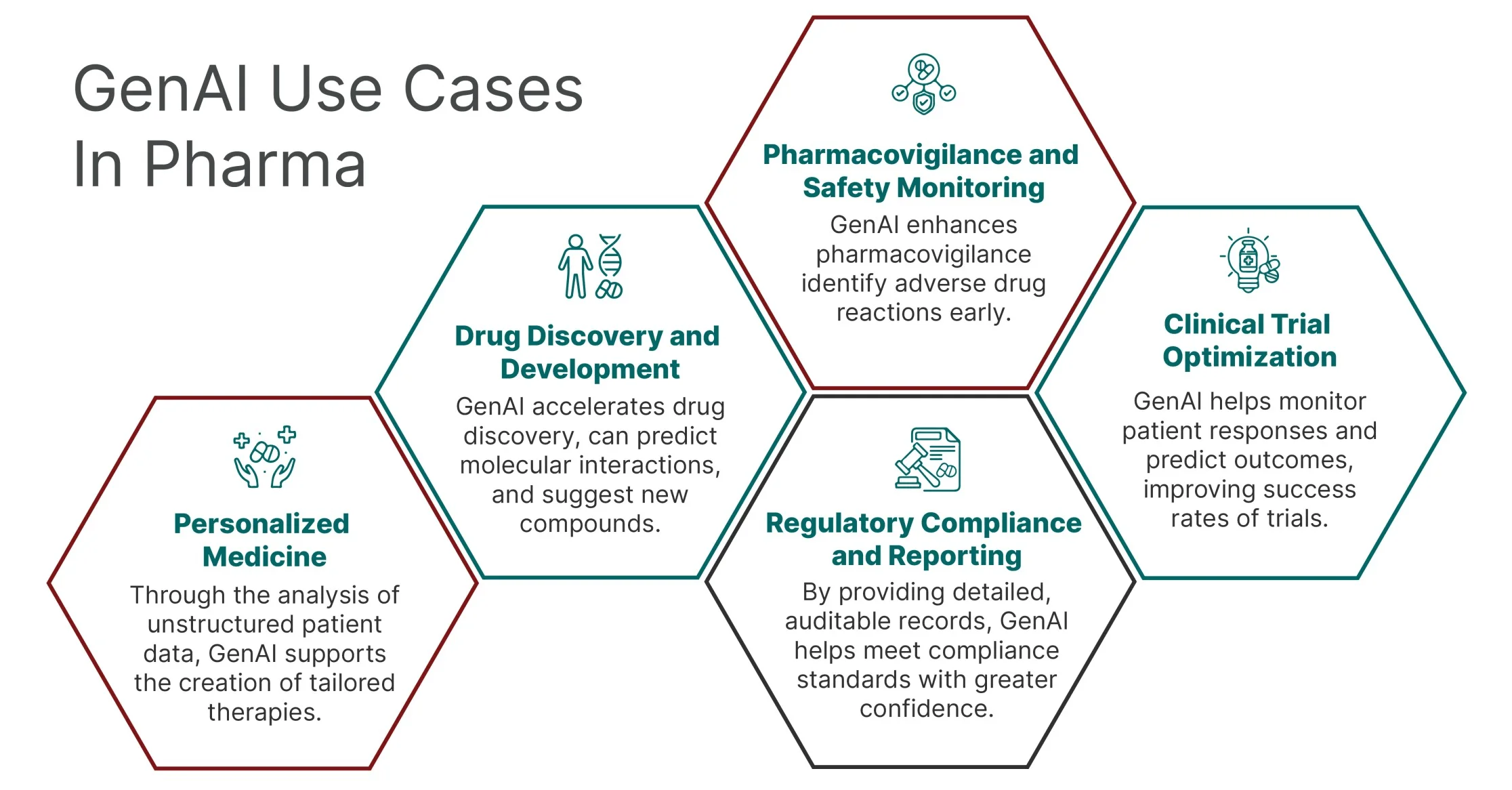
Genai use case
GenAI Best Practices and Strategic Considerations
To ensure the successful implementation of GenAI, it’s crucial to address data privacy, ethical compliance, and ROI. Given that bad data (i.e., incomplete, inaccurate, biased, outdated) leads to bad AI models, maintaining high data quality is non-negotiable in the pharma industry.
- Compliance with Regulatory Frameworks: Healthcare data is sensitive, and compliance with regulations like the EU AI Act and GDPR is essential. AI models must be transparent, auditable, and secure to protect patient information and maintain trust.
- Leveraging Specialized Models and Local Hosting: Using GenAI models trained in medical knowledge and locally hosting them enhances data security and model accuracy. It also minimizes the risk of data breaches and ensures that AI models are tailored to the complexities of healthcare data.
- Continuous data quality management (DQM) and Governance: Businesses must ensure they have laid out a strong data governance foundation. It includes continuous auditing, testing and iterating. Companies must also consider updating their data annotation and cleanup processes, and ensure queries are tested.
- Maximizing ROI Through Efficiency: Automating data structuring tasks with GenAI enhances the quality of AI models. Tracking KPIs such as reduced processing costs and faster insight generation can help quantify the impact of GenAI, ensuring it delivers both financial and patient-centric benefits.
GenAI’s ability to process and structure unstructured data is transforming RWE studies, providing clearer insights that drive better decision-making. By addressing the challenges of unstructured data and implementing GenAI with robust DQM practices effectively, pharmaceutical companies can develop AI models that are both reliable and impactful, ultimately improving patient care and outcomes.
Mu Sigma is at the forefront of integrating GenAI into healthcare data management. Our focus is on ensuring that your AI models are built on high-quality data, driving better insights and outcomes.
Contact us to learn how we can help you leverage GenAI to transform your RWE studies.


